Analysis and Research
Research
The Registry has used its data and tools to support research and studies that promote better outcomes for people with IBD. See below for examples of our studies or click here for a full list of our published papers and presentations.
Drug Safety
The Registry also established an observational drug safety programme using bespoke pages on the WebTool for data capture. The programme provided valuable pharmacovigilance information on newly introduced biosimilars.
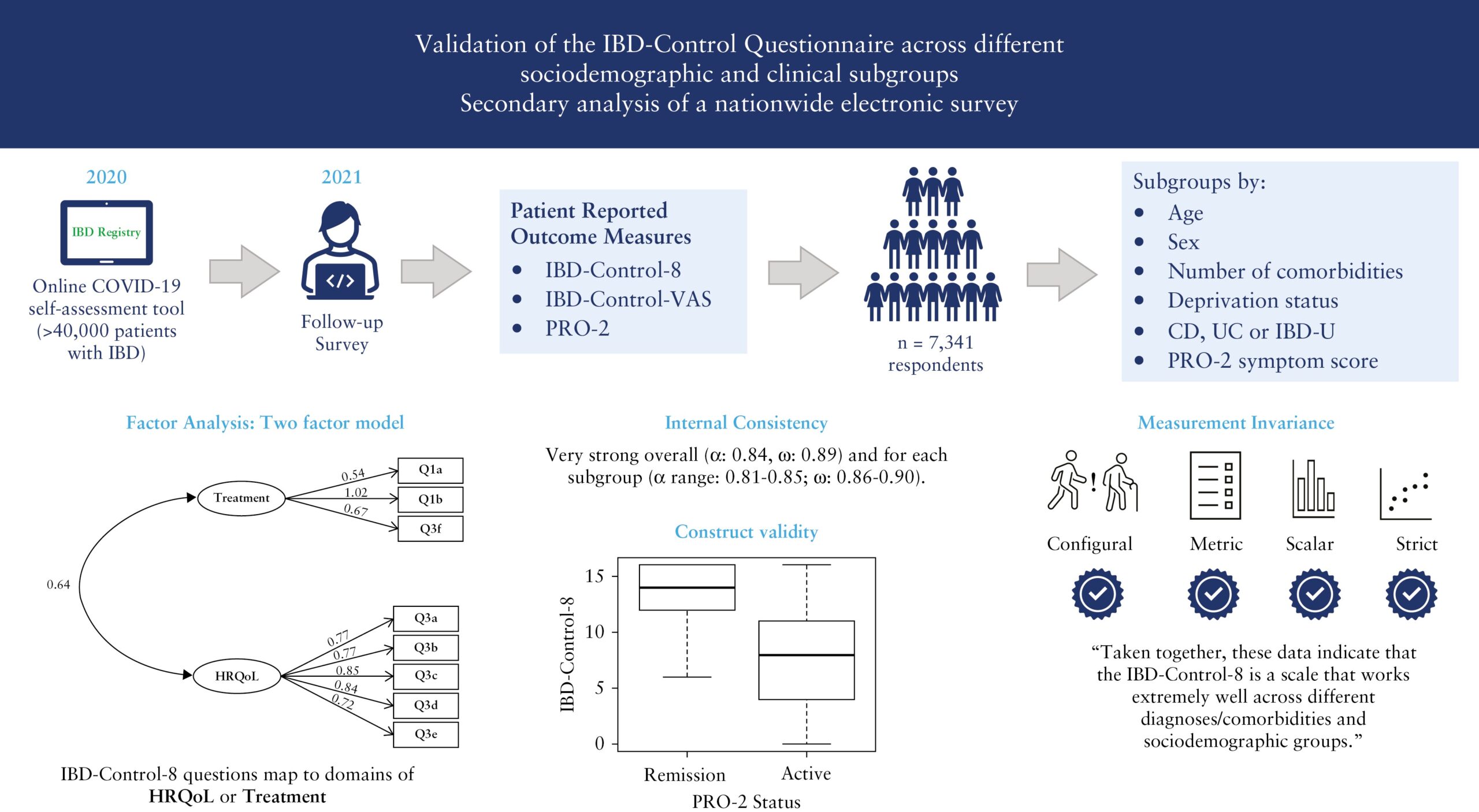
Validation of the IBD-Control Questionnaire across different sociodemographic and clinical subgroups: secondary analysis of a nationwide electronic survey
(Journal of Crohn’s & Colitis, September 2023)
This research study looks at whether the IBD-Control Questionnaire, which is a way for patients to self-assess their disease, can be used effectively in clinical practice and research. The data used were the responses to the IBD Registry’s Covid-19 IBD Risk Tool follow-on survey which patients were invited to complete after completing the IBD Risk Tool. Thank you to all those who participated in the survey.
Establishing key performance indicators for inflammatory bowel disease in the UK
(Frontline Gastroenterology, May 2023)
The IBD Registry is proud to have worked closely with the BSG IBD Section to deliver this major revision of the clinical KPIs for IBD in the UK. These KPIs will enable IBD services to make a measurable difference to patients by improving the safety, effectiveness and experience of care being delivered. The ability to monitor and benchmark services can help support patient-centred healthcare, as well as guide teams towards meaningful QI targets.
Exploring patient preferences for remote versus face-to-face appointments
(British Society of Gastroenterology Annual Meeting 2022)
Many people with IBD have had appointments over the phone or on video call since the start of the COVID-19 pandemic. Our Academic Lead, Dr Keith Bodger, is conducting a study looking at whether people with IBD prefer virtual or face-to-face appointments. We are also examining the different factors that might be linked to those preferences, such as age and whether someone’s IBD is active or not. Dr Bodger presented early findings of this study at the BSG’s Annual Meeting in June 2022. We hope these findings will help to shape future services that better suit patients’ preferences for care.
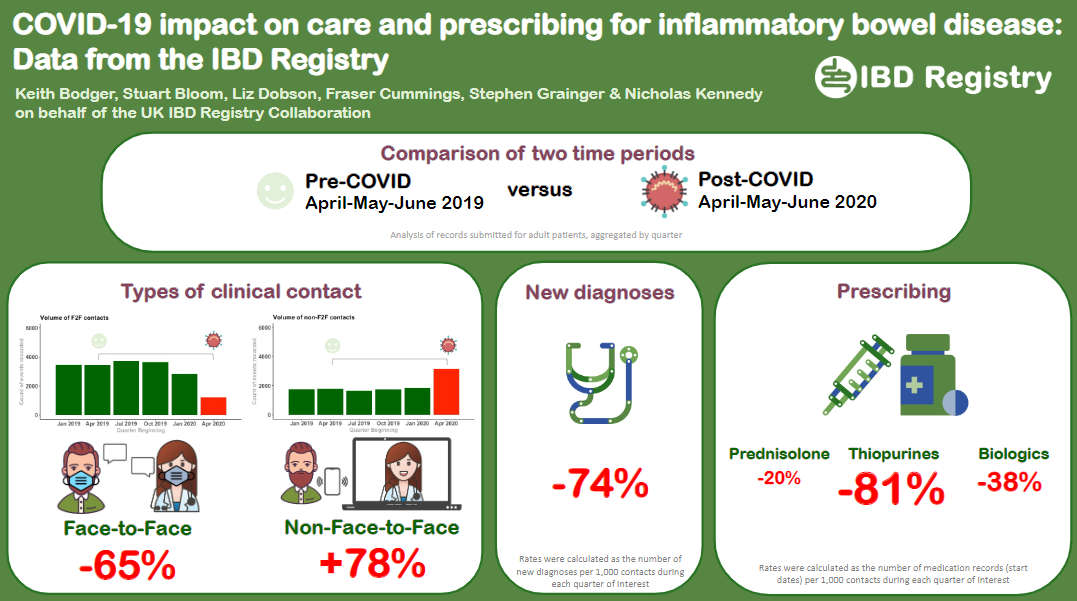
Impact of COVID-19 on IBD care
(British Society of Gastroenterology Annual Meeting 2021)
We are working with IBD hospital teams to explore the impact of the COVID-19 pandemic on care and prescribing for people with IBD.
This poster on our analysis of Registry data collected during April to June 2020 (immediate post-COVID period) and the corresponding period in 2019 (pre-COVID) was presented at the BSG’s Annual Meeting in November 2021.
Exploring take-up of the COVID-19 vaccine in people with IBD
A new study will use patient data from the IBD Registry’s COVID-19 research survey to explore levels of vaccine uptake in people with IBD in the UK. Researchers will look at factors that might influence whether people with IBD are reluctant to have the vaccine, which will help identify patient sub-groups who may benefit from education or reassurance about the vaccine and inform relevant public health programmes.
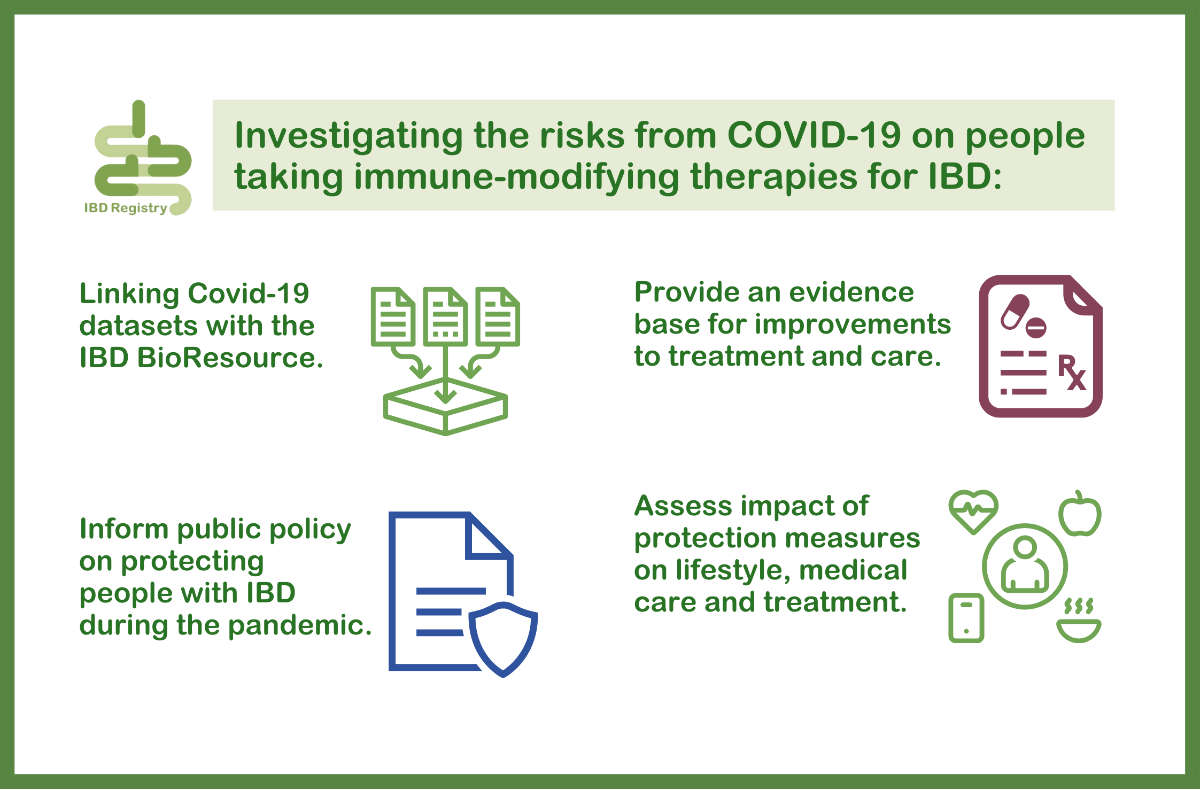
Investigating the risks from COVID-19 for people taking immune-modifying therapies for IBD
We are working with the NIHR BioResource to link our separate COVID-19 datasets together, to allow for an even greater depth of research. A new study will access both these datasets together to investigate the risk of people on immune suppressing medication experiencing severe effects from COVID-19.
This research will also look at the impact of measures such as self-isolation on lifestyle, medical care and treatment. By bringing the datasets together, our researchers can analyse lots of different types of data about people with IBD to better understand the impact of COVID-19 on their lives, build an evidence base for improvements to care, and inform public policy.
The PINPOINT study
The Prospective Incidence of Paediatric-Onset Inflammatory bowel disease in the United Kingdom
PINPOINT has started in June 2021.

The number of children newly diagnosed with inflammatory bowel disease (IBD) each year has been increasing worldwide. Some smaller regions of the United Kingdom (UK) have previously published their own incidence data for paediatric IBD. However, the last UK-wide incidence study was completed in 1999, therefore new data is urgently required. The PINPOINT study, led from Edinburgh and supported by Guts UK/BSPGHAN, will record each new diagnosis of IBD in children under 16 years of age across the whole of the UK over a 18 months. Data entry will be performed by uploading patient details to a secure platform (under the banner of the IBD Registry). The study will provide important information about the number of patients in each area of the UK, allowing the NHS and IBD charities to provide better care. This improvement in care will be driven by a better understanding of what resources are needed and how they can be used in a more beneficial way.
The PINPOINT study started in June 2021 and is being led by Dr Paul Henderson of the University of Edinburgh. The Registry is pleased to work with Dr Henderson and his team to support delivery of this project. An abstract for PINPOINT was accepted for poster presentation at ECCO 2024.
Steroids Usage
(BSG ‘Campus’ Jan 2021)
We are continuing our analysis into the use of steroids, alongside the use of biological therapies.
This poster on our analysis of the usage of steroids in IBD was presented at the BSG’s ‘Campus’ in January 2021.
The presentation is summarised in the accompanying publication:
The IBD Registry as a platform for steroid therapy audit: time trends in treatment duration (Gut, Jan 2021)
COVID-19: Risk of infection, outcomes and impact for IBD patients
Date: May-June 2021
The IBD Registry has received ethical approval to run a study on COVID-19: risk of infection, outcomes and impact for IBD patients.
We invited participants of the original IBD Risk Tool to a second survey, with additional permissions for research. We will provide a summary of the study and any updates on it on this website.
VEST (Vedolizumab Real Life Experience Study in Inflammatory Bowel Disease )

The UK Vedolizumab Real Life Experience Study in Inflammatory Bowel Disease (VEST) aims to gather outcomes data on vedolizumab in a real-world clinical setting. Hospitals across the UK participated in the study and eligible patients had to be adults who were due to start vedolizumab in standard care. Data was obtained from the patient’s own reported outcomes, clinical assessments and the medical notes including disease characterisation, medications, previous history and blood test results.
The VEST study is being led by Dr Fraser Cummings of the University of Southampton, and the Registry is pleased to work with Dr Cummings and his team to support delivery of this project. The VEST study started in 2017 and has now completed its data collection phase. An abstract for VEST was accepted for poster presentation at ECCO 2024.
Audit results
Audit of biological therapy for inflammatory bowel disease: results from the UK IBD Registry
(Gut, June 2019)
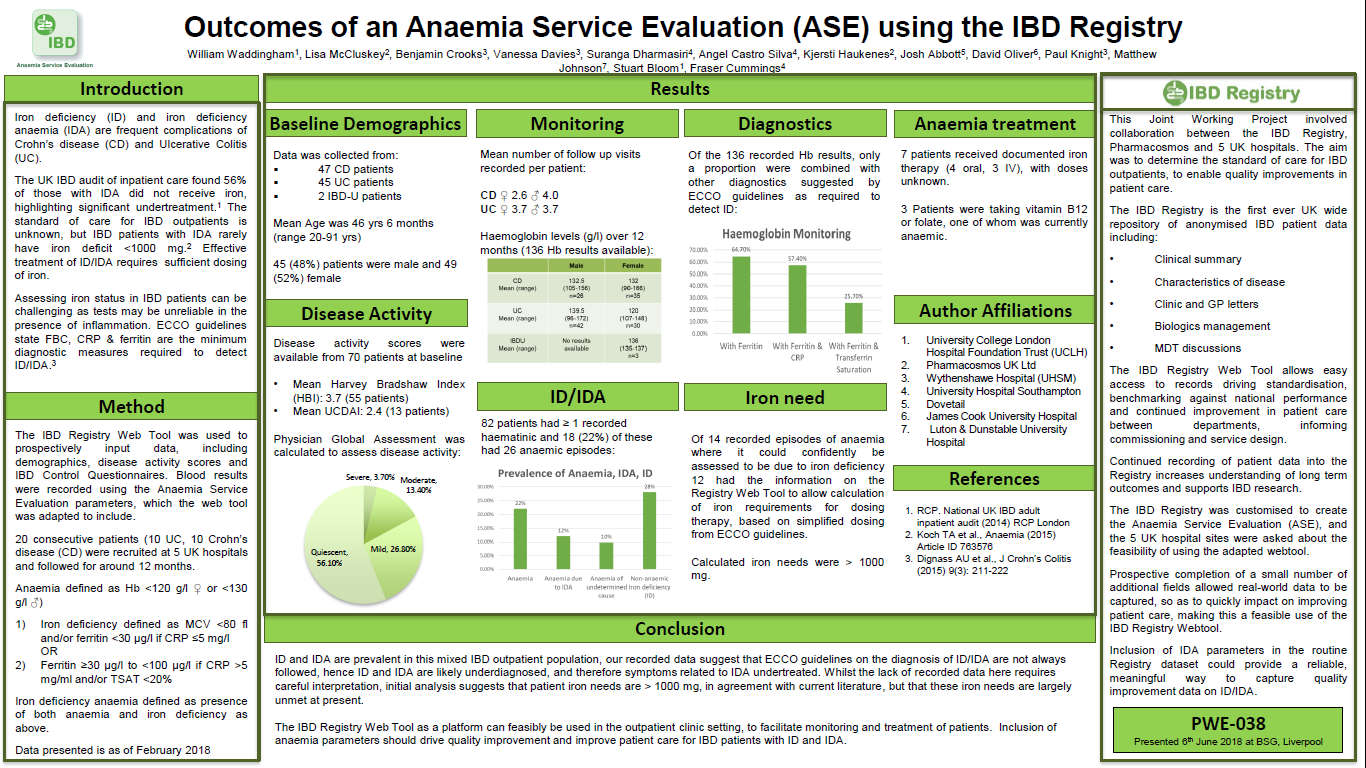
Outcomes of an Anaemia Service Evaluation using the IBD Registry
(British Society of Gastroenterology General Meeting 2018)
Iron deficiency (ID) and iron deficiency anaemia (IDA) are frequent complications of IBD. A joint working project – Anaemia Service Evaluation – with the IBD Registry and Pharmacosmos, was established to determine the level of service that patients with IBD-related ID and IDA receive and to establish the quality of treatment provided.
Data was collected on nearly 100 patients at five hospitals. In this mixed outpatient IBD population, ID was found in 23 (28%) patients, of whom the majority (78%) had IDA. The IBD Registry WebTool, adapted to record anaemia parameters, was found to be a suitable platform to facilitate the monitoring and treatment of patients.
This poster was presented at the BSG Annual General Meeting in June 2018.