Quality Accounts in England
We are delighted to announce that the IBD Registry biologics audit has been included by NHS England in Quality Accounts for 2018/19. We encourage all sites to participate in the biologics audit, as it will help to ensure that IBD patients receive a consistent standard of quality care. For the full list of Quality Accounts audits for 2018/19 please visit their website.
Data and reporting
It’s great to see the numbers continuing to rise steadily. As of the 18th January 2018, the Registry contained 34,144 patient records submitted by 55 hospitals.
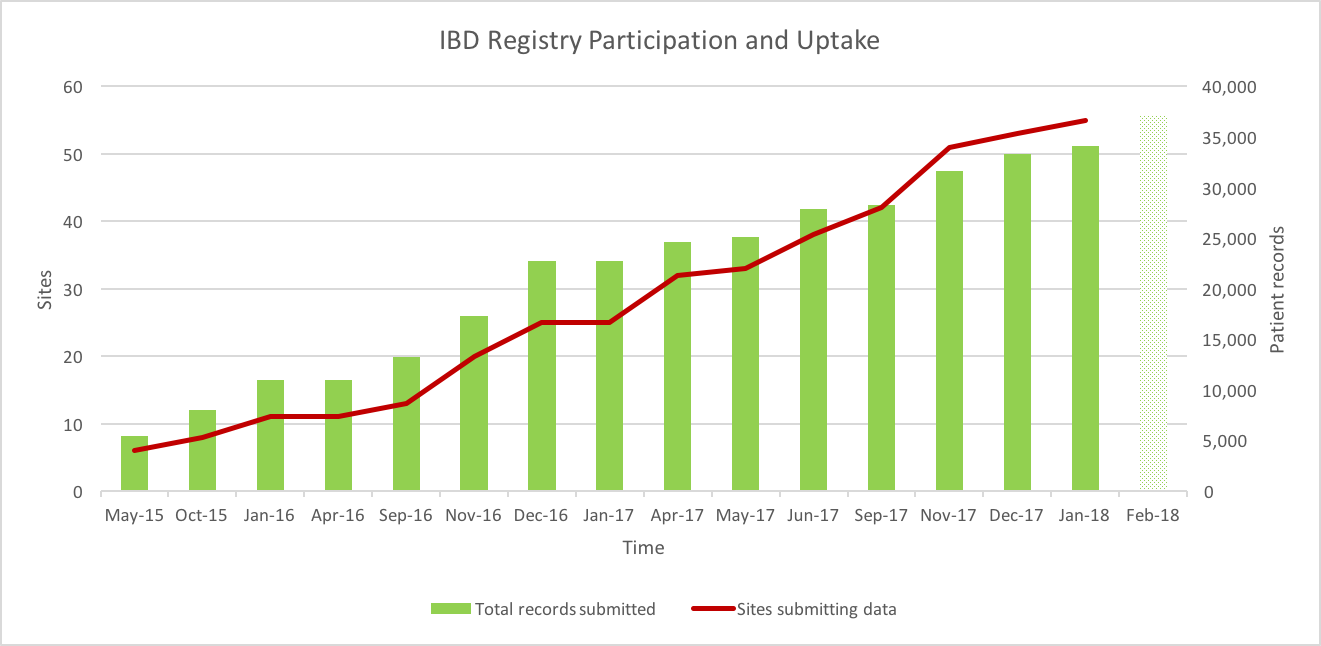
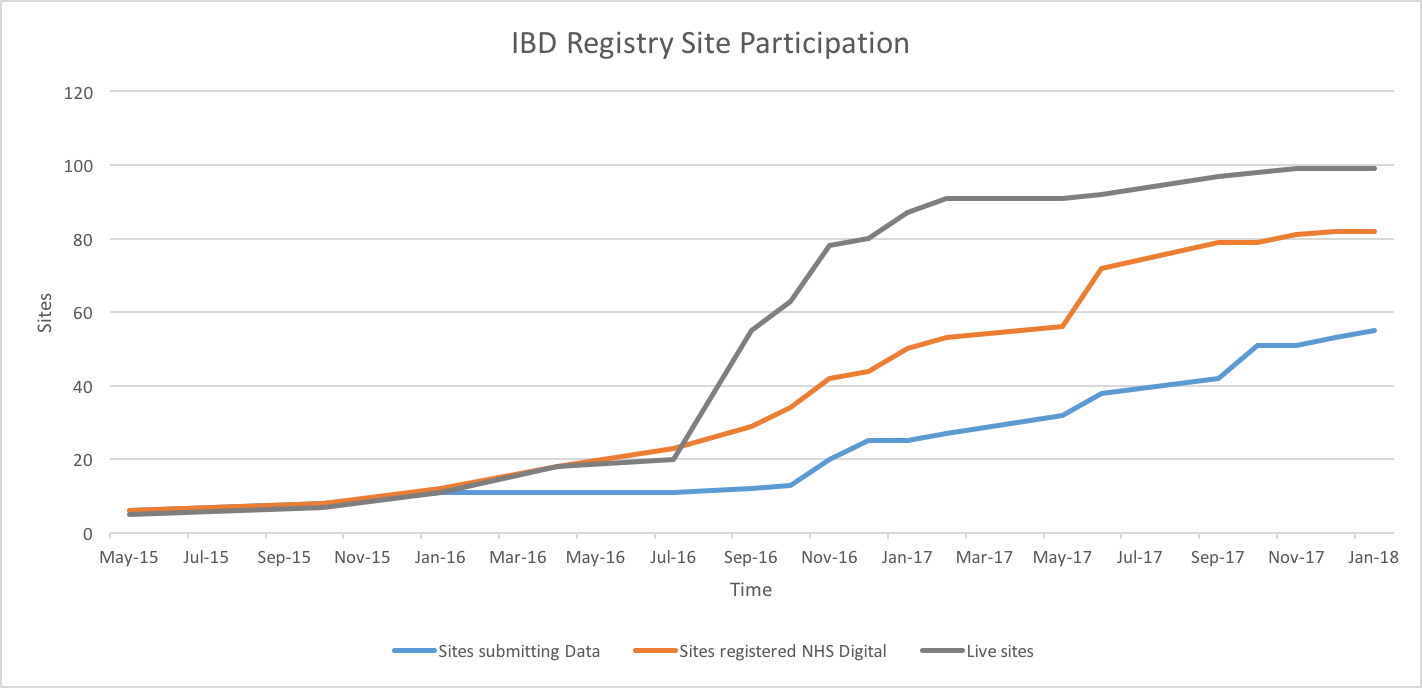
Data submission deadline
The next submission deadline will be Friday 30th March 2018. If you need help, please contact [email protected].
Other news
Mark Allan
The IBD Registry’s Data Manager Mark Allan has moved to a new position with the consumer organisation Which? Mark has been an integral member of the team over the past two years, and sites have benefited from his advice and assistance in setting up their data entry systems and uploading data. Mark also created the InfoFlex PMS User Group which has consolidated and prioritised a list of desirable changes. They will now continue to work with CIMS to implement these changes and ensure InfoFlex is a system that works for clinicians in clinic. The Registry team wish Mark success in his new job, and thank him for his dedication and commitment to the Registry. Support and other queries should still be directed to [email protected].
IBD Registry 2018 Roadshow
The IBD Registry team is considering running a roadshow in 2018, but we’d like to check it would be useful to you. Previous roadshows have allowed us to gain a better understanding of local needs, answer queries directly and share best practice. To help us plan a roadshow that ticks all the right boxes for you, we’d really appreciate your taking 4-5 minutes to complete this questionnaire https://www.surveymonkey.co.uk/r/VZVWK5F
Are you working to improve your biologics patients pre-treatment screening rates?
The 2016 Biologics Audit reported that only 60% of adults and 47% of paediatric patients received all pre-treatment screening tests. The IBD audit team at the Royal College of Physicians conducted telephone interviews with low, middle and high achievers to explore the reasons behind poor screening rates. The primary reasons for clinical teams falling short of 100% pre-treatment screening were: sites opting out of tests, difficulties collecting TB tests and obtaining results in a timely manner.
IBD teams should:
- Ensure a clear patient pathway /process is written up and visible so that the IBD team is reminded about which tests need to be conducted for pre-treatment screening prior to starting biological therapy.
- Consider who is the best professional to lead the process.
- Work with the relevant parties to improve the process for TB tests.
- Implement early pre-treatment screening of newly diagnosed patients – in particular those who may be identified as more likely to need biological therapies and/ or at higher risk of TB.
- Review national guidelines before opting out of full screening tests.
Remember hospital teams can view how well they are doing with pre-treatment screening tests compared to the standards by using the IBD Registry data visualisation tools that are available on the Registry Web tool.
Mailing list consent
Do you want to remain on the IBD Registry mailing list?
On the 25th May 2018, the General Data Protection Regulation (GDPR) will bring in new data privacy measures. To be compliant, we’ll need your written consent to hold your email address. If you want to receive future communications from the IBD Registry team, please click this link and press send. Alternatively copy the text below and send to [email protected]
“I wish to continue receiving updates from the IBD Registry.”
If you’ve already replied you don’t need to do anything. If you don’t respond before the 25th May 2018 we’ll have to take you off our contact database to ensure our compliance with the new GDPR. Please note, this is just for the monthly newsletter.
With our thanks for your continued contribution to the IBD Registry,

IBD Registry Chair
IBD Registry Clinical Lead
